Researchers at the University of Michigan have developed a catalyst material known as cobalt phthalocyanine that converts carbon dioxide—a significant driver of climate change—into renewable fuels such as methanol.
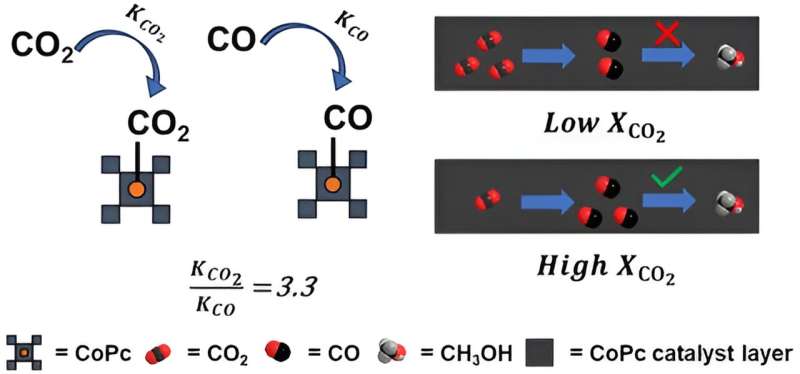
Published in the journal ACS Catalysis, U-M researchers studied using cobalt phthalocyanine as a catalyst to convert carbon dioxide into methanol through multiple reaction steps. The first step converts carbon dioxide (CO2) into carbon monoxide (CO) and the second step converts the CO into methanol.
This approach presents a sustainable method for reducing greenhouse gas emissions while offering an avenue to produce clean energy.
Scientists have long tried to find a way to chemically convert CO2 into fuels like methanol. Methanol could potentially be used to power vehicles in a more environmentally friendly way.
While the conversion of CO2 to methanol has been industrialized, achieving this transformation on a large scale through electrochemical processes has proven to be a significant challenge.
“Our approach is unique because we are able to bring and bridge all this knowledge that each field has on the same problem. We have scientists and engineers all within one team, brainstorming and gathering insights to design and understand the system in the best way possible,” said co-primary author Kevin Rivera-Cruz, who recently received a doctorate in chemistry from U-M.
Cobalt phthalocyanine acts like a molecular hook for CO2 or CO molecules. The arrangement of these molecules around the cobalt metal (the geometry) is crucial because it determines how strongly each gas molecule binds. The problem, they found, is that cobalt phthalocyanine binds much more strongly to CO2 molecules than to CO molecules. Because of this, once CO is produced in the first step, the CO is displaced by another CO2 molecule before it can be further converted to methanol.
Using advanced computational modeling, the researchers calculated that cobalt phthalocyanine binds CO2 over three times more tightly than it binds carbon monoxide. They also confirmed this through experiments measuring reaction rates when varying the amounts of CO2 and CO.
The researchers showed that the difference in binding affinity has to do with how the catalyst’s electrons interact with the CO2 and CO molecules. To solve this issue, the researchers suggest redesigning the cobalt phthalocyanine catalyst to strengthen how it interacts with CO and lessen how strongly it binds to CO2.
Resolving this roadblock could pave the way for using catalysts like cobalt phthalocyanine to efficiently convert CO2 waste into methanol fuel on a large scale.
More information:
Libo Yao et al, Electrochemical CO2 Reduction to Methanol by Cobalt Phthalocyanine: Quantifying CO2 and CO Binding Strengths and Their Influence on Methanol Production, ACS Catalysis (2023). DOI: 10.1021/acscatal.3c04957
Provided by
University of Michigan
Citation:
A leap toward carbon neutrality: New catalyst converts carbon dioxide to methanol (2024, May 6)
retrieved 8 May 2024
from https://phys.org/news/2024-05-carbon-neutrality-catalyst-dioxide-methanol.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

