CO2 photolysis experiment
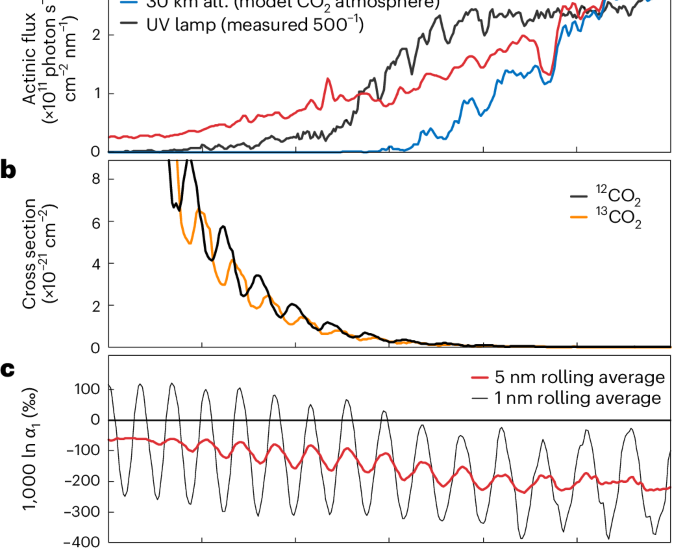
Photolysis experiments were carried out in a glass flask apparatus with a UV-grade synthetic quartz window used in ref. 16. The diameter of the UV window was 3.6 cm. The volume of the apparatus was 448 cm3, and maximum path length was 13 cm. A Xenon arc lamp (Cermax, CX-04E, PE300BUV) was used as the UV source. The actinic UV spectrum was measured by a vacuum UV monochromator system44 (Extended Data Fig. 1), which was used for calculating theoretical fractionation factor for the CO2 photolysis.
Before the experiment, the glass flask was evacuated to below 0.1 Pa for more than 12 h. After evacuation, high-purity CO2 (>99.99995%, Japan Air Gases) was introduced into the flask through the vacuum line to avoid contamination with air. The total gas pressure in the flask was measured using a capacitance manometer (MKS Baratron 626 B, 1–10 kPa range).
UV irradiation was performed at an output power of 20 A for the Xe lamp. The temperature of the flask was maintained at 315 ± 5 K using a cooling bath (AsOne MC-1). Before and during irradiation, a small aliquot of gas in the flask was sampled by expanding it to a calibrated volume with a syringe port. Each sample contained 1.7% of the total gas in the flask.
Carbon isotope analysis
The carbon isotopic composition of CO was measured using gas chromatography-combustion-isotope ratio mass spectrometry. The sampled gas was first purified by gas chromatography (Trace GC Ultra, Thermo Fisher Scientific) equipped with a capillary column (HP-MOLSIEVE:30 m × 0.53 mm i.d., 25 µm film thickness; Varian) to separate N2, O2, CO, CH4 and CO2. Ultra-high-purity He (>99.99995%, Japan Air Gases) was used as the carrier gas. The gas chromatography (GC) oven was maintained at 50 °C with a flow rate of 1.5 ml min−1. After the analysis, the GC temperature was raised to 200 °C to remove any remaining gases in the column. Purified CO was then converted into CO2 at 1,100 °C in a combustion furnace consisting of a ceramic tube packed with CuO, NiO and Pt wires. Then, CO2 was introduced into an isotope ratio mass spectrometer (MAT253, Thermo Fisher Scientific) via the combustion furnace and continuous flow interface (GC Combustion III, Thermo Fisher Scientific). Isotopic standardization was performed using CO2 injections calibrated against the National Institute of Standards and Technology natural gas standard, NGS-2.
Carbon isotope ratio is reported using the delta notation:
$${\delta}^{13}{\rm{C}}-{\rm{CO}}=\left({\,}^{13}R_{{\rm{CO}}}/{\,}^{13}R_{{\rm{CO}}2}-1\right)\,\times 1,000\,{{\permil}}$$
where 13RCO and 13RCO2 represent 13C/12C ratios of the sampled CO and the initial CO2 used in the experiment, respectively. The initial CO2 used in the photolysis experiment was also measured using GC-isotope ratio mass spectrometry (IRMS), as described in ref. 45. On the basis of replicated analyses of the samples and in-house standard CO gas, the reproducibility of the measured δ13C–CO value was better than ±0.5‰. All the experimental results are summarized in Extended Data Tables 1 and 2.
Theoretical calculation for absorption cross sections for 12CO2 and 13CO2
The absorption cross sections for 12C16O16O, 13C16O16O and three other isotopologues of CO2 were calculated from first principles using a time-dependent quantum mechanical methodology. The calculations were based on potential energy surfaces for the ground and lower excited states of CO2, which were calculated using the multireference configuration interaction plus the Davidson correction to correlation energy (MRCI + Q) quantum chemistry method (ref. 17 provides details). The absorption cross sections for 12C16O16O, 13C16O16O, and three other isotopologues of CO2 between 120 K and 395 K are available in the supporting information of ref. 17, in which Dataset S1 is for 12C16O16O, S2 is for 12C16O17O, S3 is for 12C16O18O, S4 is 13C16O16O and S5 is for 13C16O18O.
Calculation of the fractionation factor for CO2 photolysis in the experiment
In the CO2 photolysis experiment, carbon isotope fractionation potentially originates from the following two reactions:
$${{\rm{CO}}}_{2}+{{h}}{{\nu }}(\lambda < 210\,{\rm{nm}})\to {\rm{CO}}+{\rm{O}}$$
(R1)
$${\rm{CO}}+{\rm{O}}+{{M}}\to {{\rm{CO}}}_{2}+{{M}}$$
(R2)
where M represents the third-body reaction partner. The reaction rates of reactions (R1) and (R2) are proportional to the number densities of CO2 and CO and thus can be written as J[CO2] and k’[CO], respectively. In our experiment, more than 99.7% of CO2 remained in the system, and thus the reaction rate of reaction (R1) (that is, J[CO2]) was constant, whereas the reaction rate of (R2) (that is, k’[CO]) increased in response to elevated [CO] (Fig. 2). In this case, the isotopic fractionation factors for (R1) and (R2) are defined as
$${\alpha}_{1}={}^{13}J/{}^{12}J$$
(1)
$${\alpha}_{2}={}^{13}k{\prime} /{}^{12}k{\prime}$$
(2)
where 12J and 13J are the photolysis rate coefficients of 12CO2 and 13CO2, respectively. In addition, 12k’ and 13k are the rate constants for 12CO and 13CO, respectively. The J, k’, α1 and α2 values were estimated by fitting the observed changes in CO / (CO + CO2) and δ13C–CO (Fig. 2).
For the 33-kPa experiment (Extended Data Table 1), the best fit values for J and k’ were 4.3 × 10−7 s−1 and 1.7 × 10−4 s−1, respectively. The steady-state CO/CO2 ratio (= J / k’) was 0.0025. In reality, a longer experiment yields O2 via the following reaction.
$${\rm{O}}+{\rm{O}}+{{M}}\to {{\rm{O}}}_{2}+{{M}}$$
(R3)
When O2 accumulates in the system, the simple assumptions above are no longer applicable owing to the more complex O2 photochemistry and UV shielding by O2, both of which should affect the k’ and J values. Therefore, we report the data only before O2 accumulated in the system (up to 4 h under our experimental conditions).
In addition, the same model simulation was performed by splitting the J and k values into those for 12C and 13C species by assuming fractionation factors α1 and α2. For the 33-kPa experiment (Extended Data Table 1), the best fit values for α1 and α2 were 0.871 ± 0.001 and 1.0074 ± 0.0005, respectively. Consequently, the observed isotope fractionation (δ13C–CO from −133‰ to −121‰) is largely due to the isotope effect of CO2 photolysis (1,000 ln α1 = −138 ± 1‰) with a smaller contribution from reaction (R2) (1,000 ln α2 = +7.4 ± 0.5‰).
To confirm the small isotope effect of reaction (R2), additional experiments were performed, in which the reaction rate of reaction (R2) was enhanced by adding O2 (Extended Data Table 2). In this experiment, a 1:2:8 mixture of CO:O2:N2 was photolysed using the same UV source. After 345 min, 30% of the initial CO was consumed via reaction (R2). The remaining CO showed a relatively large scatter in its δ13C value (±4.4‰; n = 8) and did not show a significant correlation with the remaining CO fraction. These results confirmed that the isotope effect of reaction (R2) should be smaller than ±13‰ when considering a possible ±4.4‰ change at 30% consumption. This is consistent with the estimated +7.4 ± 0.5‰ isotope effect for reaction (R2).
In an actual atmosphere, photolysis generates oxygen atoms (O), although the direct recombination of CO with O to give CO2 is forbidden by the conservation of spin and is therefore very slow, allowing quantitative amount of CO to build up46. The excess oxygen is removed by the oxidation of surface minerals or escapes into space, and the atmosphere overall could be weakly reducing.
Also, in actual atmosphere, presence of H2O may change the photochemistry relative to our dry experimental condition. Photodissociation of H2O creates the OH radical, which is the dominant oxidant of CO. Therefore, in wet conditions, CO oxidation rate is faster than in a dry atmosphere13,14,15,16. On the other hand, the presence of H2O does not change the fractionation factor of CO2 photolysis (α1) unless water vapour considerably modifies the actinic UV spectra. The spectral effect is evaluated in the following sections.
Comparison to theoretical fractionation factor in the experimental condition
The observed large isotope effect in CO2 photolysis (α1 = 0.871 ± 0.001; 1,000 ln α1 = −138 ± 1‰) was compared with theoretical calculations to obtain a mechanistic understanding of this effect. Theoretically, the isotopic fractionation factor for CO2 photolysis (α1) can be calculated using equation (1) and absorption cross sections for CO2 isotopologues17
$${}^{12}J={\int }_{170}^{210}\varphi (\lambda ){}^{12}\sigma (\lambda )I(\lambda ){e}^{-\tau (\lambda )}d\lambda$$
(3)
$${}^{13}J={\int}_{170}^{210}\varphi (\lambda){}^{13}\sigma (\lambda )I(\lambda){e}^{-\tau (\lambda)}d\lambda$$
(4)
where 12J and 13J represent the photolysis rate coefficients of 12CO2 and 13CO2, respectively. φ(λ) is the quantum yield, which is assumed to be unity in the integral range from 170 nm to 210 nm. 12σ(λ) and 13σ(λ) are absorption cross sections at a wavelength λ for 12CO2 and 13CO2, respectively, as reported in ref. 17. I(λ) is the incident UV spectrum in our experimental condition, measured by the vacuum UV monochromator used in ref. 35 (Extended Data Fig. 1). The opacity term τ(λ) was calculated as follows:
$$\tau (\lambda )=\sum _{i}{\sigma }_{i}\int {\rho }_{i}(z)dz$$
(5)
where σi(λ) is the absorption cross section of the UV-shielding molecule i and \(\int {\rho }_{i}(z)dz\) represents the column density of molecule i in path length z. The gases defining the opacity term should have large cross sections in the 170–210 nm wavelength range. Potentially, the presence of O2 and CO2 (self shielding) can change the actinic UV flux, thereby changing the fractionation factor α1.
The calculation results are summarized in Extended Data Fig. 1. First, the actinic UV spectra did not change considerably from the front and rear ends of the 13-cm-long apparatus (Extended Data Fig. 1). CO2 self shielding may shift the isotope effect (1,000 ln α1) by up to −1‰ for 33-kPa CO2 and by a negligible amount for 10 kPa CO2 (Extended Data Fig. 1). UV shielding by O2 may possibly cause a larger shift of up to −4‰, even considering an improbably high O2 content (100 Pa; Extended Data Fig. 1). In our experiment, O2 was formed by reaction (R3), although its concentration was probably lower than that of CO (maximum 70 Pa: Extended Data Table 1). Conservatively, we take ±4‰ as the error derived from the uncertainty of the UV spectrum.
The uncertainty arising from temperature may cause a larger variation. Although our UV experiment started at 297 K, the temperature increased to 315 ± 5 K after 10 min. The heating of the apparatus was inevitable in the current experimental setting. On the other hand, the ab initio cross sections for the CO2 isotopologues change slightly depending on temperature17. Using the cross sections at 295 K, the estimated isotope effect (1,000 ln α1) was 9.4‰ larger than that using 320 K cross sections (Extended Data Fig. 1). We found that a lower temperature resulted in larger fractionation (in other words, a more negative 1,000 ln α1 value) with a slope of +0.51‰ K−1 from 220 K to 320 K.
Considering the above uncertainties, the theoretical fractionation factor α1 for our experimental condition (33 kPa CO2) was 0.845 ± 0.004, corresponding to −168 ± 5‰ for 1,000 ln α1, which was 30‰ more negative than the observed isotope effect of −138 ± 1‰.
The systematic 30‰ difference may not be due to uncertainties in the UV experiment, but are more likely due to inaccuracies in the ab initio potential energy surfaces used to calculate the theoretical CO2 cross sections. Precise calculations in the lower energy region of the absorption cross section are particularly difficult. On the basis of the 12J and 13J spectra (Extended Data Fig. 1), the lower energy part (that is λ > 183 nm) is the main region giving rise to large fractionation. Considering the difficulty of the calculation, a conservative estimate of the error in the ab initio cross sections is ±50‰, which is similar to the theoretical fractionations calculated for similar gases, including nitrous oxide (N2O)47 and carbonyl sulfide (OCS)48. Therefore, we estimate that the experimentally determined isotope effect (−138 ± 1‰) is consistent with the theoretical fractionation within an uncertainty of ±50‰. Note that calculated fractionation using theoretical cross sections17 overestimates fractionation by 30‰ relative to the experiment. The ab initio calculation and the experiment confirm our hypothesis that broadband UV photolysis of CO2 is associated with a large negative carbon isotope effect in excess of −100‰.
Estimated fractionation factor for CO2 photolysis in the early Mars atmosphere
In the early Martian atmosphere, the isotope effect in CO2 photolysis was larger than that observed in the laboratory. Considering the above fractionation mechanism, isotopic fractionation changes depending on the following key parameters:
-
1.
Temperature
-
2.
Actinic UV spectrum
-
3.
UV shielding by atmospheric species
All three of these factors from the early Mars were different from the experimental conditions and are not precisely known. Therefore, we built a 1D atmospheric simulation model to help understand the sensitivity and error.
The base model assumed a hydrostatic CO2 atmosphere with 10 mbar PCO2 at the surface (Extended Data Fig. 2a). The vertical temperature profile had a temperature of 240 K at the surface (dashed line in Extended Data Fig. 2a), which agrees with observations of the current Martian atmosphere49. A warmer case (300 K at the surface) was also calculated in the model (solid line in Extended Data Fig. 2a) to simulate early Mars with liquid water present on the surface.
The actinic UV spectra were calculated assuming the solar UV spectrum from ref. 43 and UV attenuation by CO2 at each altitude (Extended Data Fig. 2d). The UV scattering by CO2 was also considered using the scattering cross section from ref. 50.
The photolysis rates for 12CO2 and 13CO2 were calculated using equations (3), (4) and (5) (Extended Data Figs. 2b,e), which gives the isotopic fractionation factor (α1) at each altitude from equation (1) (Extended Data Fig. 2c). To evaluate the temperature dependence, we used 12CO2 and 13CO2 cross sections at each temperature of the altitude profile17. A lower temperature results in a larger calculated fractionation with a slope of +0.8‰ K−1, which is similar to, but slightly steeper than, that obtained under experimental conditions using a Xe lamp as a UV source ( + 0.5‰ K−1). Considering the systematic shift from theoretical to experimental fractionation observed, the 1,000 ln α1 value was corrected with a + 30‰ shift across the temperature range.
Estimated carbon isotope ratio for volcanic CO2 and CO of Mars
We employed a δ13C value of −25 ± 5‰ for mantle-derived volcanic CO2 and CO (Fig. 3). This value was derived from stepwise heating experiments of shergottite–nakhlite–chassignite (SNC) meteorites51. Because SNC meteorites are igneous rocks that are interpreted to represent either lavas or plutonic cumulates52, high-temperature releases of SNC meteorites would record magmatic volatiles. The high-temperature releases (>1,000 °C) of shergottites (basalt: Shergotty and Zagami), nakhlites (clinopyroxenite: Nakhla, Lafayette, Governador Valadares) and chassignite (dunnite: Chassingy) yielded a limited δ13C range (−20‰ to −30‰), despite the fact that these meteorites have distinct formation and Mars ejection ages; that is, they were formed by different magmatic activities and were derived from different launching sites on Mars53,54. The limited δ13C values (−20‰ to −30‰) suggest the existence of a common carbon reservoir in the Martian interior.
Estimated carbon isotope ratios for 4 Ga Mars surface water component and atmospheric CO2
We employed a δ13C value of +30 ± 10‰ for the dissolved inorganic carbon component of 4 Ga surficial water. This value is derived from ~4 Ga carbonates contained in the Allan Hills (ALH) 84001 Martian meteorite19,20,21. ALH 84001 is an igneous cumulate (orthopyroxenite) that experienced post-magmatic aqueous alteration, resulting in the precipitation of secondary phases including carbonate55,56. Radiogenic isotope systematics (Lu–Hf, Pb–Pb and Rb–Sr) of this meteorite yield the magmatic crystallization age and the carbonate formation age of 4.09 ± 0.03 Ga and 3.9–4.0 Ga, respectively57,58. Carbon and oxygen isotope studies indicate that carbonates were formed by distillation and loss of CO2 from the fluid during evaporation and degassing20,59 resulting in zoned isotopic enrichment of δ18O and δ13C from the core to the rim19,60,61,62,63. Triple oxygen isotope analyses further implied that the CO2 fluid could have been initially in equilibrium with the atmosphere21,64. The δ13C value of +30 ± 10‰ employed in this study was determined to cover a reported δ13C range from 20‰ to 40‰ for Ca- and Fe-rich carbonates, which are interpreted as representing near-primary precipitates in ALH 84001 (ref. 21).
A notable feature of ALH 84001 is that the carbonates are accompanied by polycyclic aromatic hydrocarbons, nanocrystal magnetites and iron sulfides because their petrographic context and textures (for example, chain-like structure of magnetite similar to magnetotactic bacteria) invoked a possible sign of life on Mars. A question related to the biogenic origin of carbonate–magnetite–sulfide assemblages is whether they formed at a sufficiently low temperature that was suitable for life. A variety of formation temperatures from 0 °C to 650 °C have been proposed for the ALH 84001 carbonates19,20,59,60,61,62,63,65,66,67,68, but recent stable isotopic studies including clumped isotope geothermometry have led to a consensus that the ALH 84001 carbonates formed in a low-temperature environment (~20 °C) (for example, ref. 20). Although the low-temperature condition is consistent with both abiotic and biogenic origins, the carbonate–magnetite–sulfide assemblage in ALH 84001 is now explained as arising from abiotic processes (for example, thermal decomposition of iron carbonate due to impact-induced shock heating)69,70.
Assuming isotopic equilibrium between carbonate and atmospheric CO2 (1,000 ln αcarbonate-CO2 = 9.8‰ at 25 °C; ref. 71), we employed a δ13C value of +20 ± 10‰ for atmospheric CO2 on early Mars (Fig. 3).
Mass balance model
Carbon isotope ratios of atmospheric CO2 (δ13CCO2) and CO (δ13CCO) were calculated based on the mass balance shown in Fig. 3c. In this simple mass balance model, we assume that the atmospheric CO2 arises primarily from volcanic input (δ13CCO2-i = −25 ± 5‰ as noted above) and removed as organics synthesized from CO. For simplicity, carbon isotopic fractionation (α) is assumed to occur only in the CO2 photolysis. Then, isotopic compositions of atmospheric CO2 is determined by remaining fraction (f) of CO2 after the photolysis:
$$\delta {}^{13}{\rm{C}}_{{\rm{CO}}2}=(1,000+\delta {}^{13}{\rm{C}}_{{\rm{CO}}2\mbox-{{i}}})\times {f}^{\;\alpha -1}-1,000$$
Owing to mass balance:
$$\delta {}^{13}{\rm{C}}_{{\rm{CO}}2\mbox-{{i}}}=f\delta {}^{13}{\rm{C}}_{{\rm{CO}}2}+(1-f\ )\delta {}^{13}{\rm{C}}_{{\rm{org}}}$$
isotopic compositions of atmospheric CO is:
$$\delta {}^{13}{\rm{C}}_{{\rm{CO}}}=(1,000+{\delta }^{13}{{\rm{C}}}_{{\rm{CO}}2\mbox-{{i}}})\times (1-{f}^{\;\alpha })/(1-f\ )-1,000$$
In this model, we do not consider carbon escape into space, which may also cause 13C enrichment of remaining CO2. Therefore, the estimated f is regarded as the maximum value if a considerable fraction of the atmospheric CO2 escapes into space. In addition, molecular diffusion in the upper part of the atmosphere may also cause isotopic fractionation. However, based on our more detailed atmospheric model31, this effect is important only above 100 km altitude, which accounts for less than 10−6 of total atmospheric CO2. Therefore, it is reasonable to neglect the isotope effect of molecular diffusion in calculating the mass balance.
Note that the model does not consider further processes that fractionate isotopes after CO2 photolysis (that is, α = α1). Therefore, the isotopic composition of the organic matter (δ13Corg) is equal to that of atmospheric CO (δ13CCO). This assumption may not be true if some organics were produced not from the CO but from the other carbon sources with normal or heavy isotopic composition. In a CO2 and/or CO atmosphere, however, organic synthesis is initiated from the HCO radical produced by CO13,16,31,35,46,72,73,74,75:
$${{\rm{CO}}}_{2}+{{h}}\nu \to {\rm{CO}}+{\rm{O}}$$
(R1)
$${\rm{CO}}+{\rm{OH}}\to {{\rm{CO}}}_{2}+{\rm{H}}$$
(R4)
$${\rm{CO}}+{\rm{H}}+{{M}}\to {\rm{HCO}}+{{M}}$$
(R5)
$${\rm{HCO}}+{\rm{HCO}}\to {\rm{HCHO}}+{\rm{CO}}$$
(R6a)
$${\rm{HCO}}+{\rm{HCO}}\to({\rm{C}}2\,{\rm{organics}})$$
(R6b)
In comparison to reaction (R1), it is very likely that carbon isotope fractionations for reactions (R4)–(R6) are an order of magnitude smaller than the CO2 photolysis31,76,77. Therefore, it is reasonable to assume the strongly 13C-depleted signature in CO should be transferred to formaldehyde and other organics. There is no known atmospheric reaction that incorporates the isotopically normal CO2 directly into organic molecules77, unless CO2 is photolysed into CO with a large isotopic fractionation (reaction (R1)). The possible alternative carbon source with normal 13C abundance is volcanic CO. Therefore, we have performed additional calculations considering 10%, 20% and 30% addition of volcanic CO (δ13CCO-i = −25 ± 5‰) and shown in Fig. 3.