Scientists in Japan have developed a new method for breaking down toxic “forever chemicals” quickly and at room temperature. The technique broke down 100% of certain types of these pollutants overnight, recovering some useful components for reuse.
Per- and polyfluoroalkyl substances (PFAS) are a broad class of chemicals that have excellent stability and resistance to water and heat, largely thanks to their strong carbon-fluorine bonds. This makes them perfect for everything from non-stick cookware to firefighting foam and water-repellant clothing.
But those super-strong bonds have a downside too – since they don’t break down, the chemicals tend to linger in the environment essentially “forever,” hence their nickname. Worse still, when they accumulate in the human body they’ve been linked to diabetes, fertility issues, various cancers, immune system disruption, and many other health conditions.
Arima et al, Angew. Chem. Int. Ed. 2024
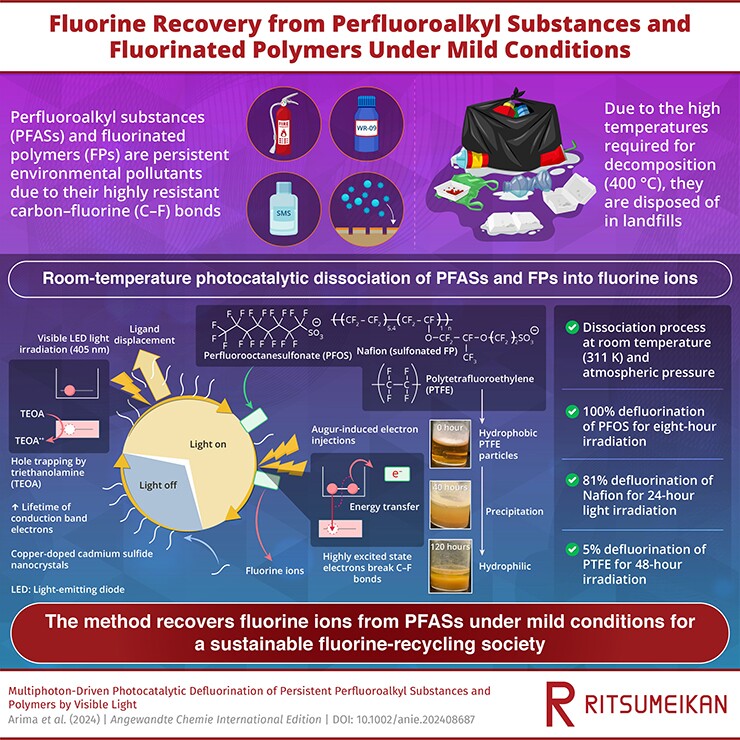
Now, scientists at Ritsumeikan University in Japan have developed a new method for breaking down PFAS. Semiconductor nanocrystals of cadmium sulfide (CdS), some of which are doped in copper, are the active ingredients in a solution that also contains water, a compound called triethanolamine (TEOA), and of course the PFAS chemicals waiting to be treated.
When the solution is exposed to LED lights at wavelengths of 405 nm, the nanocrystals become excited and cause the PFAS molecules to stick to their surface. At the same time, electrons are generated and excited in the solution, until they remove fluorine ions from the PFAS molecules, breaking those sturdy bonds.
In tests, this method successfully broke down 100% of a particular PFAS, called perfluorooctanesulfonate, in just eight hours. Another, called Nafion, broke down by 81% in 24 hours. This was achieved at a temperature of just 38 °C (100 °F) – far cooler than the 400 °C (752 °F) usually required. The technique also recovers the fluroine ions, allowing them to be reused for other industrial applications.
The technique is similar to many others that use a catalyst to break down PFAS molecules, but usually UV light is required and often a higher temperature. Other teams have found success with similar reactions using supercritical water, magnetic particles, hydrogen or boron nitride. Ultimately, having a mix of options for breaking down PFAS could be the best solution.
The research was published in the journal Angewandte Chemie International Edition.
Source: Ritsumeikan University

