A team led by Lawrence Berkeley National Laboratory (Berkeley Lab) has invented a technique to study electrochemical processes at the atomic level with unprecedented resolution and used it to gain new insights into a popular catalyst material.
Electrochemical reactions—chemical transformations that are caused by or accompanied by the flow of electric currents—are the basis of batteries, fuel cells, electrolysis, and solar-powered fuel generation, among other technologies. They also drive biological processes such as photosynthesis and occur under the Earth’s surface in the formation and breakdown of metal ores.
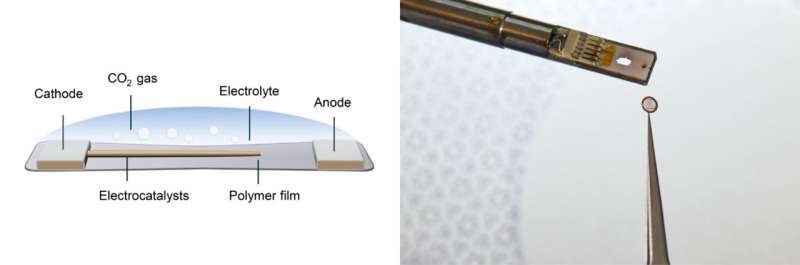
The scientists have developed a cell—a small enclosed chamber that can hold all the components of an electrochemical reaction—that can be paired with transmission electron microscopy (TEM) to generate precise views of a reaction at an atomic scale. Better yet, their device, which they call a polymer liquid cell (PLC), can be frozen to stop the reaction at specific timepoints, so scientists can observe composition changes at each stage of a reaction with other characterization tools.
In a paper appearing in Nature, the team describes their cell and a proof of principle investigation using it to study a copper catalyst that reduces carbon dioxide to generate fuels.
“This is a very exciting technical breakthrough that shows something we could not do before is now possible. The liquid cell allows us to see what’s going on at the solid-liquid interface during reactions in real time, which are very complex phenomena. We can see how the catalyst surface atoms move and transform into different transient structures when interacting with the liquid electrolyte during electrocatalytic reactions,” said Haimei Zheng, lead author and senior scientist in Berkeley Lab’s Materials Science Division.
“It’s very important for catalyst design to see how a catalyst works and also how it degrades. If we don’t know how it fails, we won’t be able to improve the design. And we’re very confident we’re going to see that happen with this technology,” said co-first author Qiubo Zhang, a postdoctoral research fellow in Zheng’s lab.
Zheng and her colleagues are excited to use the PLC on a variety of other electrocatalytic materials, and have already begun investigations into problems in lithium and zinc batteries. The team is optimistic that details revealed by the PLC-enabled TEM could lead to improvements in all electrochemical-driven technologies.
New insights into a popular catalyst
The scientists tested the PLC approach on a copper catalyst system that is a hot subject of research and development because it can transform atmospheric carbon dioxide molecules into valuable carbon-based chemicals such as methanol, ethanol, and acetone. However, a deeper understanding of copper-based CO2 reducing catalysts is needed to engineer systems that are durable and efficiently produce a desired carbon product rather than off-target products.
Zheng’s team used the powerful microscopes at the National Center for Electron Microscopy, part of Berkeley Lab’s Molecular Foundry, to study the area within the reaction called the solid-liquid interface, where the solid catalyst that has electrical current through it meets the liquid electrolyte. The catalyst system they put inside the cell consists of solid copper with an electrolyte of potassium bicarbonate (KHCO3) in water. The cell is composed of platinum, aluminum oxide, and a super thin, 10 nanometer polymer film.
Using electron microscopy, electron energy loss spectroscopy, and energy-dispersive X-ray spectroscopy, the researchers captured unprecedented images and data that revealed unexpected transformations at the solid-liquid interface during the reaction.
The team observed copper atoms leaving the solid, crystalline metal phase and mingling with carbon, hydrogen, and oxygen atoms from the electrolyte and CO2 to form a fluctuating, amorphous state between the surface and the electrolyte, which they dubbed an “amorphous interphase” because it is neither solid nor liquid. This amorphous interphase disappears again when the current stops flowing, and most of the copper atoms return to the solid lattice.
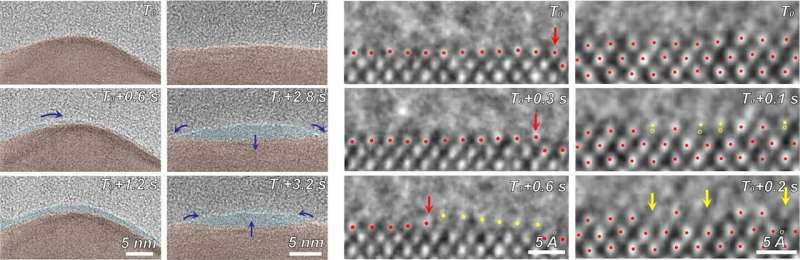
According to Zhang, the dynamics of the amorphous interphase could be leveraged in the future to make the catalyst more selective for specific carbon products. Additionally, understanding the interphase will help scientists combat degradation—which occurs on the surface of all catalysts over time—to develop systems with longer operational lifetimes.
“Previously, people relied on the initial surface structure to design the catalyst for both efficiency and stability. The discovery of the amorphous interphase challenges our previous understanding of solid-liquid interfaces, prompting a need to consider its effects when devising strategies,” said Zhang.
“During the reaction, the structure of the amorphous interphase changes continuously, impacting performance. Studying the dynamics of the solid-liquid interface can aid in understanding these changes, allowing for the development of suitable strategies to enhance catalyst performance,” added Zhigang Song, co-first author and postdoctoral scholar at Harvard University.
The other authors on this work were Xianhu Sun, Yang Liu, Jiawei Wan, Sophia. B. Betzler, Qi Zheng, Junyi Shangguan, Karen. C. Bustillo, Peter Ercius, Prineha Narang, and Yu Huang.
More information:
Haimei Zheng, Atomic dynamics of electrified solid–liquid interfaces in liquid-cell TEM, Nature (2024). DOI: 10.1038/s41586-024-07479-w. www.nature.com/articles/s41586-024-07479-w
Provided by
Lawrence Berkeley National Laboratory
Citation:
New technology provides electrifying insights into how catalysts work at the atomic level (2024, June 19)
retrieved 20 June 2024
from https://phys.org/news/2024-06-technology-electrifying-insights-catalysts-atomic.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.

